Ionic Bonds
n chemical bonds, atoms can either transfer or share their valence electrons. In the extreme case where one or more atoms lose electrons and other atoms gain them in order to produce a noble gas electron configuration, the bond is called an ionic bond.Typical of ionic bonds are those in the alkali halides such as sodium chloride, NaCl.
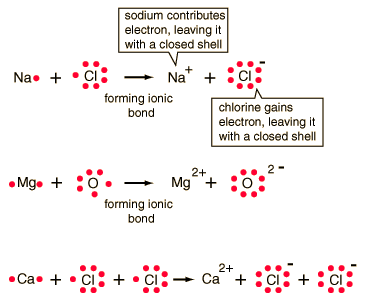 | Ionic bonding can be visualized with the aid of Lewis diagrams. |

No comments:
Post a Comment